 胡可课题组
胡可课题组
Research Synopsis
Research Synopsis
The research of our photochemistry group focuses on solar energy as one of the most promising
forms of renewable energy and aims to solve the global crisis of climate change concerning our
human survival. We aim to transform cheap and ubiquitous substances including H2O, CO2, N2, or
lignin into valuable fuels or value-added chemicals such as H2, CO, CH3OH, NH3, etc with
sunlight and some “chemical magic”. One of the most important advantages for the students being
trained in our research group is getting to learn the “chemical magic”, a.k.a. the understanding
of fundamental electron transfer processes through state-of-the-art spectroscopic techniques in
our laboratory. Students and Postdocs in our photochemistry group will be immersed in the
following research topics:
1. Energy-demanding organic photoredox catalysis and the underlying photochemistry of visible
light harvesting molecular photocatalysts
2. Solar fuels production and the underlying photoinduced electron transfer mechanisms
3. Photoelectrochemical devices for artificial photosynthesis
Our independent research is also made possible through very fruitful collaborations:
· Gerald J. Meyer, University of North Carolina at Chapel Hill (UNC), USA
· Renato N. Sampaio, University of North Carolina at Chapel Hill (UNC), USA
· Ludovic Troian-Gautier, UCLouvain, Belgian
· Matthew V. Sheridan, Soochow University, China
· Jianli Hua, East China University of Science and Technology, China
· Baojiang Jiang, Heilongjiang University, China
· Jia Guo, Fudan University, China
· Zhang-Jie Shi, Fudan University, China
Ongoing Research Projects
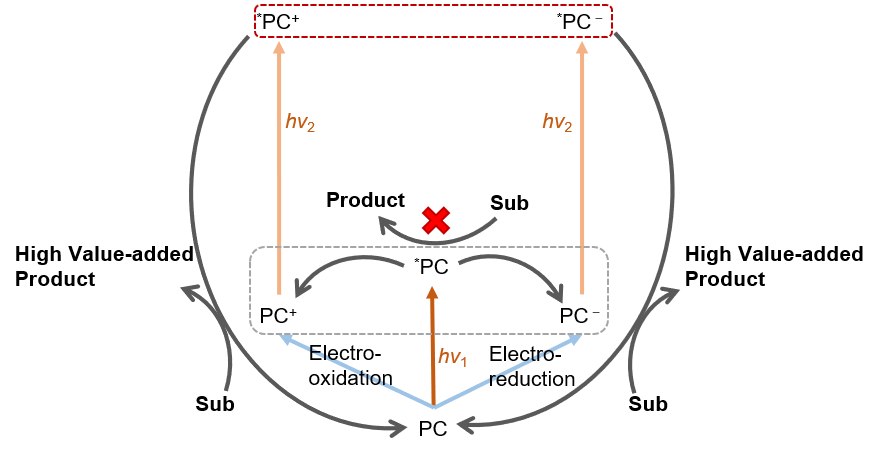
Ongoing Research Projects 1. Consecutive Light Excitation Approach to Energy-Demanding Synthesis of Value-Added Chemicals Inert chemical bonds such as C-H, C-Cl, N≡N, etc. require highly reducing or oxidizing reagents (E < -2 V or> 2 V vs. SCE) to initiate chemical transformations to value-added chemicals. Excited states of typical photocatalysts such as ruthenium or iridium based metal complexes are incapable of achieving such extreme redox potentials. Our research group set out to use small organic photocatalysts that could consecutively absorb multiple photons and reach super-photooxidants or reductants. Our current achievements include using N-phenylphenothiazine (PTH) photocatalyst to do one electron oxidation of chloride and activate C(sp3)-H bonds for the synthesis of functionalized alkanes; using hybrid structure of perylene diimide (PDI) with ZrO2 nanoparticles to activate aryl C-Cl bonds or CO2 reduction catalyst at low catalytic concentrations.
1) Zhao, Z.; Li, J.; Yuan, W.; Cheng, D.; Ma, S.; Li, Y.;Shi, Z.; Hu, K.*, Nature-Inspired Photocatalytic Azo Bond Cleavage with Red Light. J. Am. Chem. Soc. 2024, 146, 1364-1373.
2) Zhao, Z.; Niu, F.; Li, P.; Wang, H.; Zhang, Z.; Meyer, G. J.*; Hu, K.*, Visible Light Generation of a Microsecond Long-Lived Potent Reducing Agent. J. Am. Chem. Soc. 2022, 144, 7043-7047.
3) Li, P.; Deetz, A. M.; Hu, J.; Meyer, G. J.*; Hu, K.*, Chloride Oxidation by One- or Two-Photon Excitation of N-Phenylphenothiazine. J. Am. Chem. Soc. 2022, 144, 17604-17610.
4) Li, P.; Bourgois, C.; Glaser, F.; De Kreijger, S.; Cadranel, A.; Troian-Gautier, L.*; Hu, K.*, Outcompeting Thermodynamics: Ion-Pairing and Coulombic Interactions to Trigger Perfluoroacetate Intra-Ionic Photooxidation for Perfluoroalkylation Reactions. J. Am. Chem. Soc. 2025, 147, 12082-12091.
5) Li, P.; Zhao, Z.; Tian, L.; Liu, R.; Wang, X.; Yang, W.; Cui, Y.-S.; Humphrey, M. G.; Zhang, C.*; Hu, K.*, Harnessing the Potent Excited-State Reductive Power of Pyrene-Modified N-Heterocyclic Carbene-Stabilized Au13 Nanoclusters for Four-Electron Nitroarene Reduction with Unity Selectivity. J. Am. Chem. Soc. 2025, 147, 44817-44824.
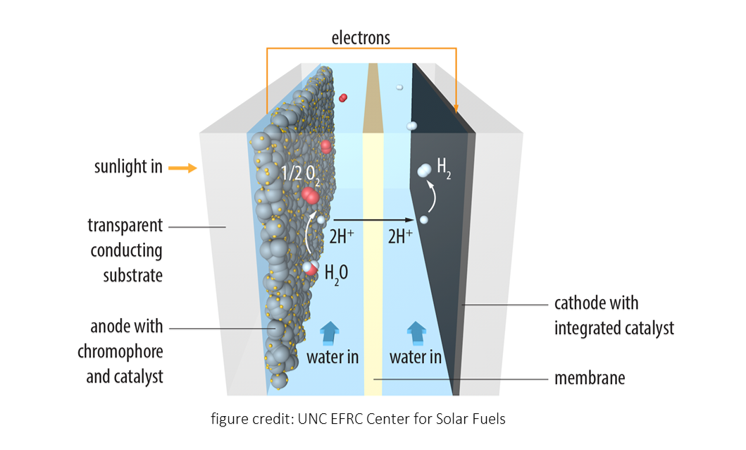
2. Photoelectrochemical Cells (PEC) for Artificial Photosynthesis (Liquid Sunlight)
The sun is the most abundant renewable energy upon which a sustainable society can be built. Currently, harvesting 1/7000 of the total solar energy reaching the surface of our earth will suffice the entire energy demand of our human society. Natural photosynthesis in which CO2, water, and sunlight are converted into carbohydrates and dioxygen gives us a perfect example to learn the trick of storing solar energy into high energy chemical bonds. Our research group set out to use visible light absorbing semiconductor based photoelectrodes for the construction of photoelectrochemical cells (PEC) to mimic natural photosynthesis. Our current efforts include using modified bismuth vanadate as the photoanode material for water, glycerol oxidation or organic C-H functionalization; using dye-sensitized photoelectrodes for the construction of tandem cells for total water splitting.
1) Hu, K.; Sampaio, R. N.; Schneider, J.; Troian-Gautier, L.; Meyer, G. J.* Perspectives on Dye Sensitization of Nanocrystalline Mesoporous Thin Films. J. Am. Chem. Soc. 2020, 142 (38), 16099-16116.
2) Niu, F.; Zhou, Q.; Han, Y.; Liu, R.; Zhao, Z.; Zhang, Z.; Hu, K.*, Rapid Hole Extraction Based on Cascade Band Alignment Boosts Photoelectrochemical Water Oxidation Efficiency. ACS Catal. 2022, 12,10028-10038.
3) Niu, F.; Zhang, P.; Zhang, Z.; Zhou, Q.; Li, P.; Liu, R.; Li, W.; Hu, K.*, Ultrathin corrugated nanowire TiO2 as a versatile photoanode platform for boosting photoelectrochemical alcohol and water oxidation. J. Mater. Chem. A, 2023, 11, 4170.
4) Han, Y.; Chang, M.; Zhao, Z.; Niu, F.; Zhang, Z.; Sun, Z.; Zhang, L.; Hu, K.*, Selective Valorization of Glycerol to Formic Acid on a BiVO4 Photoanode through NiFe Phenolic Networks. ACS Appl. Mater. Interfaces 2023, 15, 11678–11690
5) Yang, W.; Li, P.; Han, Y.; Zhao, Z.; Tian, L.; Zhang, Z.; Humphrey, M. G.; Zhang, C.; Hu, K. Two-step coupled photoelectrochemical chlorination and oxygenation of C(sp3)–H bonds mediated by chlorine radicals over a modified BiVO4 photoanode. Chem. Sci. 2025, 16, 20229-20238.
Laboratory Equipment
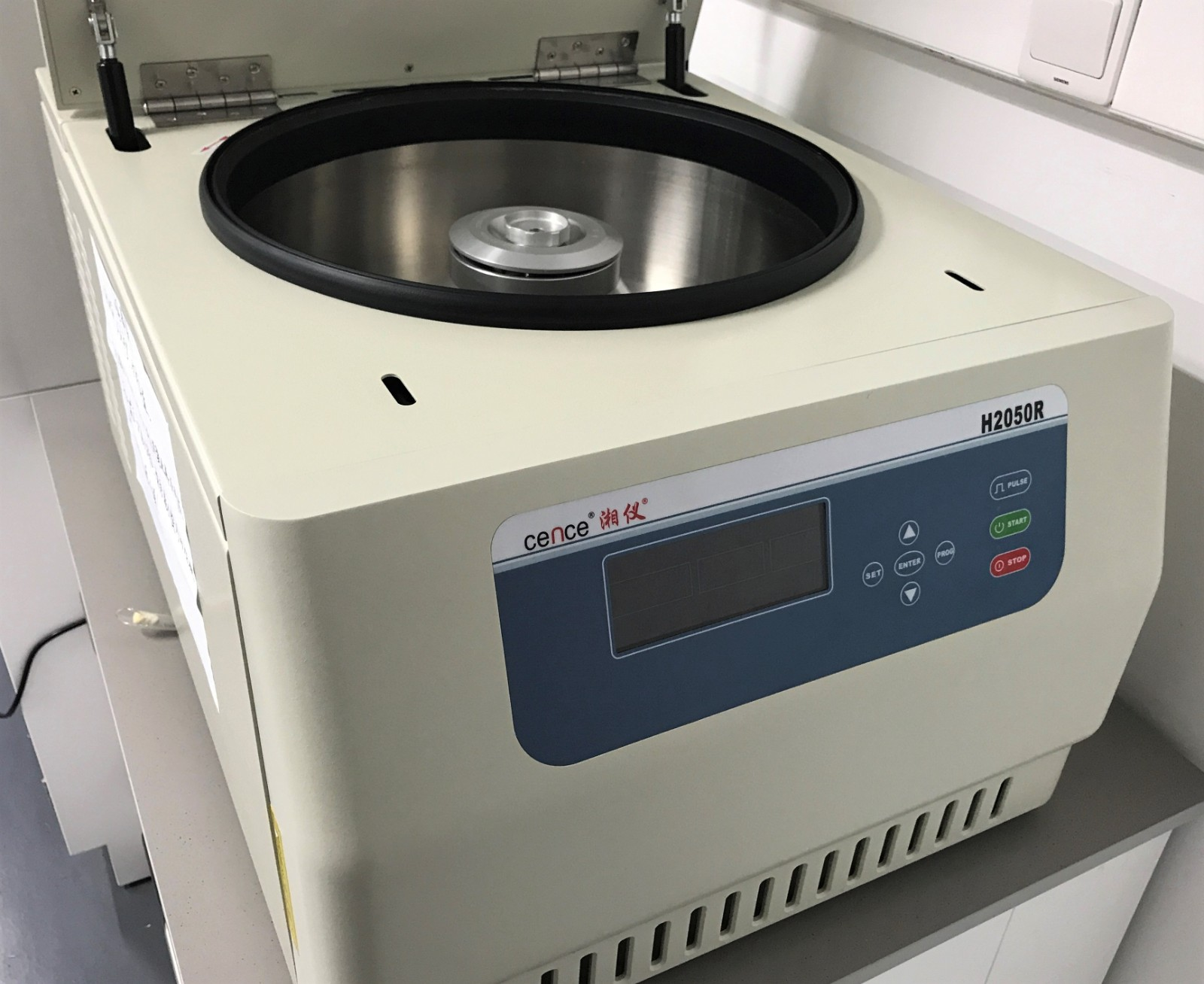
High-speed freezing centrifuge(15,000 rpm)

Fume hoods & Schlenk Line

Tube Furnace(KEJING)

Freeze drier

Agilent Cary 60 UV–Vis Spectrophotometer

TSP2000 Transient absorption spectrometer

Agilent Cary Eclpse Fluorescence Spectrophotometer*
(Provided by instrument experimental platform)

Electrochemical Workstation
(CHI760+CHI1232)

Xenon Lamp light source (AM 1.5 G)

GC9790Plus

Laser

Perfect Light Multi-channel Photoreactor