Harnessing the Potent Excited-State Reductive Power of Pyrene-Modified N-Heterocyclic Carbene-Stabilized Au13 Nanoclusters for Four-Electron Nitroarene Reduction with Unity Selectivity
摘要
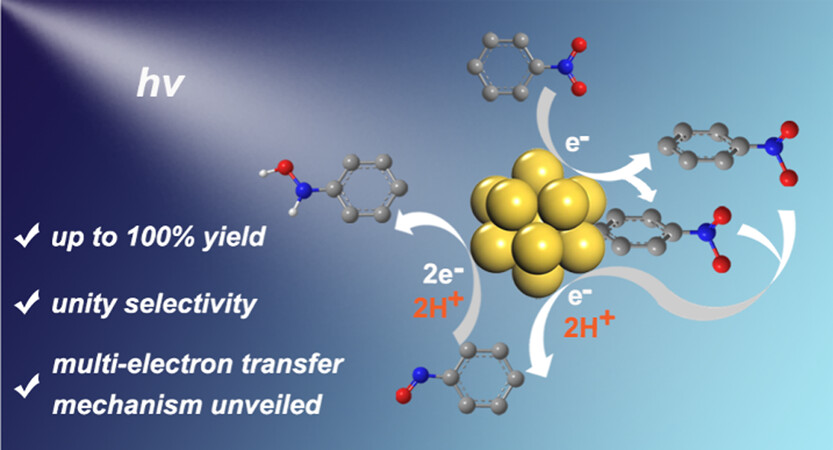
This work describes the use of a pyrene-modified N-heterocyclic carbene (NHC)-stabilized Au13 nanocluster (denoted as Au NC) as the visible light photocatalyst in the multielectron reduction of nitrobenzene (NB). The photoreduction of NB to N-phenylhydroxylamine (PHA) proceeds with unity selectivity and product yield after 3 h of irradiation. The photocatalytic reduction proceeds via a four-electron and four-proton process. NB is first reduced by the excited state of the Au NC (Au NC*) to yield the ion pair (Au NC+ and NB•–). Further excitation of Au NC within the ion pair and involvement of external protons result in the reduction of NB•– to nitrosobenzene (NSB). The diffusion-controlled electron transfer in the first step is the rate-determining step, with the combination of dynamic and static quenching in the second step expediting electron transfer from Au NC* to NB•–. The third and fourth electron transfer events that lead to the final product are much faster than the first. Density functional theory (DFT) simulations link the selective formation of PHA to its weak adsorption on Au NC, driven by hydrophobic interactions and electronic effects of the pyrene ligands of Au NC. The excited-state reduction potential of Au NC* is −1.69 V vs SCE (Saturated Calomel Electrode), comparable to those of commonly used CdS quantum dot-based photocatalysts. This NB photoreduction study has afforded valuable insights into the intricate mechanisms at NHC-stabilized gold nanoclusters in energy-demanding multielectron transfer photocatalytic reactions.